Abstract
Disease recurrence and toxicity are common sequelae of CD19-directed chimeric antigen T-cell (CAR-T) cell therapy in large B-cell lymphoma (LBCL). We have developed a machine learning approach that is informative of adverse CAR-T outcomes and can support personalized treatment approaches.
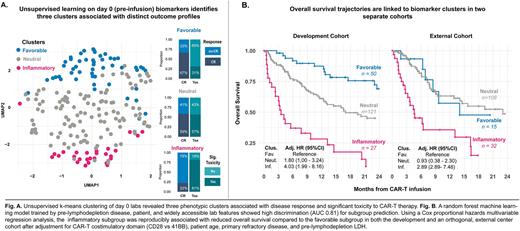
In a single-center cohort of 198 LBCL patients treated with CD19-CAR-T cells (axicabtagene ciloleucel [axi-cel] 50%, tisagenlecleucel [tisa-cel] 32%, lisocabtagene maraleucel [liso-cel] 18%), we applied unsupervised k-means clustering on standard laboratory and cytokine measurements collected within the day prior to CAR-T infusion. Three distinct biomarker clusters emerged (Fig. A) - inflammatory (n=27, 14%), neutral (n=121, 61%), and favorable (n=50, 25%). The inflammatory subgroup was enriched for patients with high levels of inflammatory markers such as IL-6, TNFa, and ferritin, while the favorable cluster tended to have higher complete blood counts and albumin; intermediate values were characteristic of the neutral subgroup.
To determine the clinical implications of these subgroups, we studied their association with day 100 best response to CAR-T, significant toxicity by day 30 (defined as life-threatening complications such as immune toxicities requiring intervention, bloodstream infections, or early death), and overall survival (OS) after multivariate adjustment for CAR-T costimulatory domain (CD28 vs 41BB), patient age, primary refractory disease, and pre-lymphodepletion LDH. Compared to those in the favorable biomarker cluster, patients in the inflammatory cluster (Fig. A) had increased odds of not achieving CR by day 100 (odds ratio [OR] 4.23, 95% confidence interval [CI] 1.37-14.4, p < 0.05) and a high toxicity profile (OR 12.5, 95% CI 3.66-49.5, p < 0.001). OS was also reduced with the inflammatory subgroup in univariable analysis (Fig. B) and a multivariable Cox regression model (hazard ratio [HR] 4.03, 95% CI 1.99-8.16, p < 0.001). Patients in the neutral subgroup, compared to those in the favorable group, had reduced overall survival (HR 1.80, 95% CI 1.00-3.24, p < 0.05) and increased odds of high toxicity (OR 3.40, 95% CI 1.56-7.79, p <0.01).
Serial assessment of the uncommon biomarkers and cytokines in our day 0 panel may not be readily available to many centers. We developed a random forest prediction model for cluster type based on patient, disease, and widely available pre-lymphodepletion laboratory features. Our random forest model outperformed gradient boosting and logistic regression model alternatives and achieved a high discrimination (AUC 0.81) for cluster prediction. Next, we applied the random forest model to pre-lymphodepletion data from an independent LBCL cohort (n=155) from a different center (Fig. B). Most patients were assigned to the neutral subgroup (n=108, 70%) followed by the inflammatory (21%) and favorable (9%) subgroup. Assignment to the inflammatory cluster was strongly associated with inferior OS compared to the favorable subgroup (HR 2.89, 95% CI 1.12-7.48, p < 0.05) after multivariate adjustment for CAR-T costimulatory domain (CD28 vs 41BB), patient age, primary refractory disease, and pre-lymphodepletion LDH.
In conclusion, we identified three pre-infusion (day 0) biomarker clusters, which are tightly associated with CAR-T outcomes. These clusters could guide decision-making regarding hospitalization after CAR-T infusion and to preempt early inflammation and disease relapse in high-risk subgroups. Finally, to improve accessibility to cluster assignment, we developed a prediction model for cluster type based on readily available pre-lymphodepletion data and applied it towards an external center cohort as a proof-of-principle.
Disclosures
Perales:Vor Biopharma: Honoraria; Cidara Therapeutics: Consultancy; VectivBio AG: Honoraria; Takeda: Honoraria; Medigene: Consultancy; Servier: Consultancy; Bellicum: Honoraria; DSMB: Other; Astellas: Honoraria; Celgene: Honoraria; Karyopharm: Honoraria; MorphoSys: Consultancy, Honoraria; Orca Bio: Consultancy; Omeros: Consultancy; Abbvie: Honoraria; Sellas Life Sciences: Consultancy; Novartis: Honoraria; Nektar Therapeutics: Consultancy, Honoraria; Miltenyi Biotec: Consultancy, Honoraria; Merck: Consultancy; Kite, a Gilead Company: Honoraria, Research Funding; Incyte: Honoraria, Research Funding; Bristol-Mysers Squibb: Honoraria. Batlevi:ADC Therapeutics: Other: Provision of Services; Bristol-Myers Squibb: Other: Ownership / Equity Interests; Provision of Services; Dava Oncology: Other: Provision of Services; Autolus: Research Funding; Bayer: Research Funding; Epizyme: Research Funding; Janssen: Research Funding; Novartis: Research Funding; Roche/Genentech: Research Funding; Xynomic: Research Funding; GLG Pharma: Consultancy; Juno/Celgene: Consultancy; Kite Pharma: Consultancy; Life Sciences: Consultancy; Seattle Genetics: Consultancy. Giralt:Kite: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; MILTENYI: Research Funding; TAKEDA: Membership on an entity's Board of Directors or advisory committees, Research Funding; OMEROS: Research Funding; SPECTRUM Pharma: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Actinuum: Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz Pharmaceutical: Membership on an entity's Board of Directors or advisory committees; Johnson & Johnson: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding. Jacoby:Novartis: Membership on an entity's Board of Directors or advisory committees. Palomba:BeiGene: Consultancy; Ceramedix: Consultancy. Salles:Roche/Genentech, Gilead Sciences, Janssen, Celgene, Novartis, MorphoSys AG, Epizyme, Alimera Sciences, Genmab, Debiopharm Group, Velosbio, Bristol-Myers Squibb, BeiGene, Incyte, Miltenyi Biotec, Ipsen, Kite, a Gilead Company, Loxo, Rapt: Consultancy; AbbVie, BeiGene, Bristol Myers Squibb, Celgene, Debiopharm, Epizyme, Genentech/Roche, Genmab, Incyte, Kite, a Gilead Company, Miltenyi, MorphoSys, Takeda, and VelosBio: Membership on an entity's Board of Directors or advisory committees; Roche/Genentech, Janssen, Celgene, Gilead Sciences, Novartis, AbbVie, MorphoSys AG, Amgen, Bayer, Epizyme, Regeneron, Kite, a Gilead Company: Honoraria. Scordo:Medscape, LCC (CME): Honoraria; i3Health (CME): Honoraria; Amgen, Inc.: Research Funding; Omeros Corporation: Consultancy, Research Funding; Angiocrine Bioscience, Inc.: Consultancy, Research Funding; Kite - A Gilead Company: Other: Ad-hoc advisory board (past); McKinsey & Company: Consultancy. Shah:Amgen: Research Funding; Beyond Spring: Research Funding; Janssen: Research Funding. Slingerland:Seres Therapeutics: Other: salary support through a sponsored agreement. Avigdor:Takeda, Gilead, Novartis, Roche, BMS: Consultancy; AbbVie: Honoraria. van den Brink:Rheos Medicines: Honoraria; Vor Biopharma: Honoraria; Pluto Therapeutics: Current holder of stock options in a privately-held company, Honoraria; Notch Therapeutics: Current holder of stock options in a privately-held company, Honoraria; Frazier Healthcare Partners: Honoraria; Nektar Therapeutics: Honoraria; Ceramedix: Honoraria; Lygenesis: Honoraria; GlaskoSmithKline: Honoraria; Da Volterra: Honoraria; Thymofox: Honoraria; Garuda: Honoraria; Novartis (Spouse): Honoraria; Synthekine (Spouse): Honoraria; Beigene (Spouse): Honoraria; Kite (Spouse): Honoraria; Juno Therapeutics: Other: IP Licensing ; DKMS: Other: fiduciary role on the Foundation Board ; Wolters Kluwer: Patents & Royalties; Seres Therapeutics: Current holder of stock options in a privately-held company, Honoraria, Other: IP Licensing , Research Funding. Shouval:Medexus: Consultancy, Ended employment in the past 24 months; MyBiotics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal